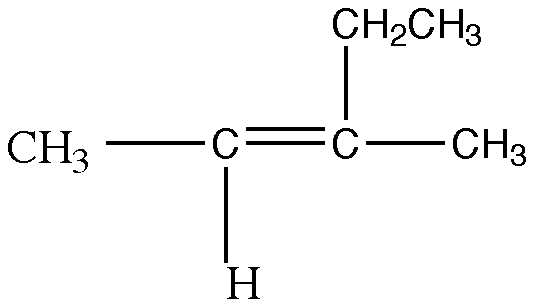
1. Name the following compound using the IUPAC nomenclature system:

a) 2-ethyl-2-butene
b) 2-ethylbutane
c) 3-methyl-2-pentene
d) 3-methyl-3-pentene
e) 2,2-ethyl-2-butene
2. Why do aromatic compounds undergo substitution reactions instead of addition reactions?
a) The aromatic ring doesn't have any C=C bonds to undergo addition reactions
b) The aromatic ring is very stable and quickly reforms if disrupted
c) Additions to C=C double bond always form carbanions
d) The total number of pi electrons must change
e) Aliphatic rings are unable to undergo addition reactions
3. Name the following compound using the IUPAC nomenclature system:
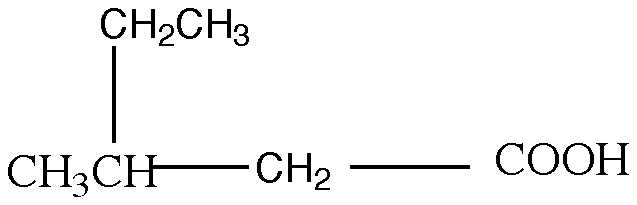
a) 2-ethylpropanoic acid
b) 3-ethylpropanoic acid
c) 3-ethylbutanoic acid
d) 2-methylpentanoic acid
e) 3-methylpentanoic acid
4. Which of the following carbohydrates can be classified asa an aldose?
a) CH2OHCHOHCHOHCH2OH
b) CH2OHCHOH(C=O)CH2OH
c) CH2OH(C=O)CH2OH
d) CHOCHOHCHOHCH2OH
e) CH2OHCH2OH
5. Which of the following amino acids has an acidic side chain?
a) glycine
b) leucine
c) methionine
d) lysine
e) glutamic acid
6. Each amino acid has two functional groups in common. These are:
a) Carboxyl and amine
b) ester and amine
c) carboxyl and amide
d) alcohol and amine
e) aldehyde and carboxyl
7. Which of the following compounds is an aldehyde?
a) propanol
b) propanoic acid
c) propanal
d) propanone
e) propene
8) The primary structure of a protein is determined by:
a) how many heterocyclic bases are present
b) the exact sequence of amino acids
c) whether the sugar unit is ribose or deoxyribose
d) whether the amino acids are neutral or acidic
e) whether only purine or pyrimidine bases are present
9. DNA and RNA differ only in that;
a) one contains deoxyribose and the other ribose
b) one contains thymine and deoxyribose while the other contains uracil and ribose
c) one contains phosphoric acid and one does not
d) one contains purine bases and one does not
e) one contains cytosine and one does not
10. DNA or RNA contain the following basic units:
a) amino acid chain, phosphoric acid, and a sugar
b) an amine base, phosphoric acid and a sugar
c) amino acid chain, an amine base and phosphoric acid
d) Amino acid chain, amine base, phosphoric acid and a sugar
e) amino acid and sugar
11. Which statement is not correct regarding the function of a catalyst?
a) it lowers the activation energy
b) it changes the mechanism of a reaction
c) it affects the rate of a chemical reaction
d) it lowers the energy of the product causing the reaction to be more exothermic
e) none of the above is incorrect
12. For second order reactions the rate constant, k, has the units of:
a) time-1
b) mol L-1 time-1
c) L mol-1 time
d) mol2 L-2 time-1
e) L mol-1 time-1
13. For first order reactions the slope of a plot of ln [A] vs. time is:
a) k
b) -k
c) kt
d) k/t
e) k ln[A0]
14. The half life for a first order reaction is known to be 4.5 h. How long will it take for 85% to be decomposed in hours?
a) 1.1
b) 9.0
c) 10
d) 12
e) 27
15. The decomposition of nitrogen dioxide to nitrogen and oxygen is second order with a rate constant, k = 12.5 M-1 s-1. If the initial concentration of NO2 is 0.0500 M, what is the [NO2] after 45.0 s?
a) 8.42 x 10-3
b) 8.42 x 10-4
c) 1.72 x 10-3
d) 8.46 x 10-2
e) 1.24 x 10-2
16. The reaction of O3 with Cl is believed to occur by the mechanism shown below. In this mechanism, Cl is:
O3 + Cl ----- ClO + O2
ClO + O ----- Cl + O2
a) The catalyst
b) An intermediate
c) A product of the overall reaction
d) A reactant in the overall reaction
e) The activated complex
17. Which is not ususually a factor in determining the rate of a reaction according to collision theory?
a) Orientation of the molecules that collide
b) Kinetic energy of the molecules that collide
c) The temperature of the reaction mixture
d) The number of particles per unit volume in the sample
e) The stability of the product
18. The t1/2 of a first order decomposition (A --- products), is 26 hours when the initial concentration of A is 0.256 M. How many hours would it take for the concentration of A to drop from 0.256 M to 0.064 M?
a) 52 hours
b) 9.2 hours
c) 78 hours
d) 36 hours
e) 26 hours
19. The rate equation for the decomposition of H2O2 is rate = k[H2O2] [I-]. What are the units of the rate constant, k?
a) time-1
b) mol L-1 time-1
c) L mol-1 time
d) L2 mol-2 time-1
e) L mol-1 time-1
20. According to the collision theory of kinetics, which statement best describes the rate of a chemical reaction?
a) All collisions result in a chemical reaction
b) All collisions between molecules with at least a minimum kinetic energy result in a reaction
c) All collisions between molecules with a kinetic energy equal to or greater than the activation energy result in a reaction
d) The greater the difference in energy between the reactants and products the faster is the reaction
e) The greater the difference in energy between the reactants and the transition state the faster is the reaction