SO2Cl2 - Sulfuryl Chloride:
First draw the Lewis dot structure:
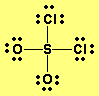
Electron geometry: tetrahedral. Hybridization: sp3
Then draw the 3D molecular structure using VSEPR rules:
Decision:
The molecular geometry of SO2Cl2 is tetrahedral with an asymmetric charge distribution on the central atom.
Therefore this molecule is polar.
Sulfuryl Chloride on Wikipedia.
Back to Molecular Geometries & Polarity Tutorial: Molecular Geometry & Polarity Tutorial.
For homework help in math, chemistry, and physics: www.tutor-homework.com.
