NOBr Nitrosyl Bromide:
First draw the Lewis dot structure:
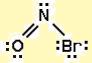
Electron geometry: trigonal planar. Hybridization: sp2
Then draw the 3D molecular structure using VSEPR rules:
Decision:
The molecular geometry of NOBr is bent (or angular) with asymmetric charge distribution on the central atom.
Therefore this molecule is polar.
Nitrosyl Bromide Wiki page.
Return to the molecular geometries: Molecular Geometry & Polarity Tutorial.
Return to the homepage: www.tutor-pages.com.
Get homework help with molecular geometry: www.tutor-homework.com.
