SiH4 - Silicon Tetrahydride, better known as Silane:
First draw the Lewis dot structure:
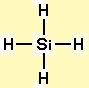
Electron geometry: tetrahedral. Hybridization: sp3
Then draw the 3D molecular structure using VSEPR rules:
Decision:
The molecular geometry of SiH4 is tetrahedral with symmetric charge distribution around the central atom.
Therefore this molecule is non-polar.
Silicon Tetrahydride on Wikipedia.
Back to Molecular Geometries & Polarity Tutorial: Molecular Geometry & Polarity Tutorial.
For homework help in math, chemistry, and physics: www.tutor-homework.com.
