VSEPR Rules:
| Electron and Molecular Geometry On Central Atom. Click for Print View. | ||||||||||||
| Electron Regions, shape, & hybridization | Bonding Regions | Lone Pairs | Electron Region Geometry | Molecular Geometry | Examples | |||||||
2 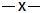 sp sp |
2 | 0 | linear | linear | BeF2, CO2 | 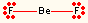 |
||||||
| 1 | 1 | linear | CO, N2 | :N≡N: :C≡O: |
||||||||
3 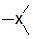 sp2 sp2 |
3 | 0 | trigonal planar | trigonal planar |
BF3, CO32- | 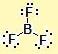 |
||||||
| 2 | 1 | bent | O3, SO2 | 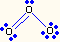 |
||||||||
| 1 | 2 | linear | O2 | 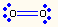 |
||||||||
4 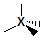 sp3
sp3 |
4 | 0 | tetrahedral | tetrahedral | CH4, SO42- | 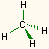 |
||||||
| 3 | 1 | trigonal pyramidal | NH3, H3O+ | 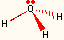 |
||||||||
| 2 | 2 | bent | H2O, ICl2+ |  |
||||||||
| 1 | 3 | linear | HF, OH- | 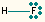 |
||||||||
5  sp3d sp3d |
5 | 0 | trigonal bipyramidal | trigonal bipyramidal | PF5 | 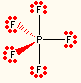 |
||||||
| 4 | 1 | seesaw | SF4, TeCl4, IF4+ | 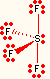 |
||||||||
| 3 | 2 | T-shaped | ClF3 | 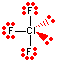 |
||||||||
| 2 | 3 | linear | I3-, XeF2 | 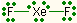 |
||||||||
6  sp3d2 sp3d2 |
6 | 0 | octahedral | octahedral | SF6, PF6-, SiF62- | 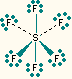 |
||||||
| 5 | 1 | square pyramidal | BrF5, SbCl52- | 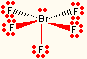 |
||||||||
| 4 | 2 | square planar |
XeF4, ICl4- | 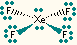 |
||||||||
Molecular Geometry & Polarity Example Problems
Remember!... Step 1: Draw the Lewis structure, Step 2: Draw the 3D molecular structure w/ VSEPR rules,Step 3: Use symmetry to determine if the molecule is polar or non-polar.
Click on the molecule's name to see the answer, but first try to do it yourself!