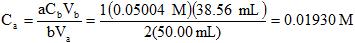Click Here for Print Version: Stoichiometry_Flowchart.jpg
Homepage Chemistry Tools, Links, & Apps
According to Wikipedia stoichiometry is the calculation of quantitative (measurable) relationships of the reactants and products in chemical reactions. For example, if I start with 10 gallons of gasoline, how many liters of oxygen gas would be required to burn the 10 gallons of gasoline? Or maybe a question such as if I have 10 grams of sugar and 10 grams of sulfuric acid, which one will be used up first (which is the "limiting reactant"). To answer such questions involving chemical reactions we always need the balanced chemical reaction that refers to the specific situation.
Let's first look at a simple example that is likely familiar to you and doesn't involve chemical reactions.
Part I. Converting from mass to volume and volume to mass: Density is our conversion factor.
Example 1. Convert 45.9 mL of liquid mercury (Hg) to grams of mercury. We need to look up the density of mercury, 13.5 g/mL.
Note: make sure that you have the right units where they belong. You want mL to "cancel out".
Well, that pretty simple, but at this point I'd like to show the flowchart and how to use it:

Click Here for Print Version: Stoichiometry_Flowchart.jpg
Wow! That's confusing! But look at the red density conversion on the flowchart above. We are converting from volume to mass, so we use density as the conversion factor.
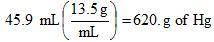
Example 2. How many mL is 1.50 kg of gasoline? If you look up the density of gasoline, it is 0.688 g/mL (pure isoocane, no ethanol).
First convert 1.50 kg to grams, and obviously that is 1.50x103g.
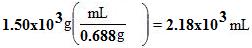
Part II. Converting from mass to moles and from moles to mass: Molar mass is our conversion factor.
Molar mass is often called molecular weight or formula weight, depending on the type of compound (molecular or ionic).
Example 3. Convert 100. g of water to moles of water.
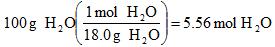
This is a simple enough calculation and I could have left out "H2O", but I find it helpful when you're converting from the mass of one compound to the mass of another, so I often include the chemical formula.
Likewise you can convert moles to grams. Try to do this one yourself:
Example 4. Convert 10.5 mole of methane, CH4, to grams of methane. The answer is 168 g CH4.
A good tool to calculate molar masses is: http://www.tutor-homework.com/Chemistry_Help/molar_mass_calculator.html.
Part III. Converting from moles of one compound to moles of another: We use the stoichiometric coefficients.
NOTE: This is likely the most important step in stoichiometric calculations, but one that students often forget or miscalculate.
Ane we ALWAYS NEED A BALANCED REACTION!!!
Example 5. How many moles of aluminum oxide can be made from 18.7 moles of Aluminum metal, assuming we have more than enough oxygen gas to react?
To first answer this, we need a balanced reaction:
4 Al(s) + 3 O2(g) --> 2 Al2O3
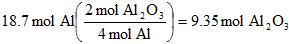
And how many moles of oxygen gas are needed ("consumed")?
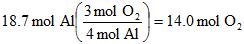
Part IV. Converting between moles and number of molecules: Use Avogadro's number, 6.022x1023.
This is very much like converting between "dozen eggs" and just eggs. If you have 10 doz. eggs, then you have 120 eggs, if you have 3.5 doz. eggs, you have 42 eggs.
Example 6. How many molecules are in 10.3 moles of hydrogen gas?
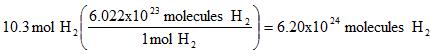
Part V. Converting between molecules and atoms. Use the molecular formula.
Example 7. We'll pick up from where we left off above. How many atoms of hydrogen are there from the step above?
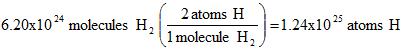
Part VI. Converting volume of a solution to the moles present: We use molarity (written M or mol/L) as the conversion factor.
Example 8. If you have 50.3 mL of 0.206 M solution of sodium chloride, how many moles of sodium chloride are there?
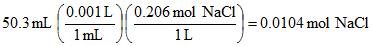
Part VII. Combining it all!
Example 9. How many atoms of gold are in 103.2 lbs of the compound [Au(CH2)2P(C6H5)2]2Cl2?
To simplify things, the condensed formula is Au2C28H28P2Cl2 and its molar mass is 891.33 g/mol.
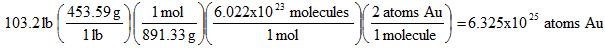
Notice that we first converted pounds to grams, then grams to moles, moles to molecules, and finally molecules to atoms. There are no shortcuts to go from the first step to the last, that's why I made that flowchart above to show how to go from one quantity to another.
Example 10. If we have 31.8 grams of hydrogen gas, and more than enough oxygen gas, how many grams of water can be made?
Grams of compound "A" to grams of compound "B". This is one of the most common types of problems!
First we need the balanced reaction! 2 H2 + O2 --> 2 H2O
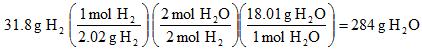
Example 11. If you have 100.0 g of Al and 100.0 g of O2, how much (in grams) aluminum oxide, Al2O3, can be made?
This is a limiting reactants a.k.a. limiting reagents (pronounced "re-agents") problem and THESE ARE DIFFICULT.
Balanced reaction!: 4 Al(s) + 3 O2(g) --> 2 Al2O3(s)
These are the steps to follow:
(a) Convert each reactant to moles:
100.0 g Al (1 mol / 26.98 g) = 3.706 mol Al
100.0 g O2 (1 mol / 32.00 g) = 3.125 mol O2
(b) Divide the moles by their stoichiometric coefficients in the balanced reaction. (This is how I tell which reactant is the limiting reactant).
Al: 3.706 / 4 = 0.9265
O2: 3.125 / 3 = 1.042
You can see that the aluminum has the smallest mole/stoichiometric coefficient ratio; therefore aluminum is the limiting reactant!
(c) The amount of products made and reactants remaining all depends on the limiting reactant.
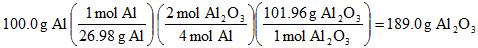
Example 12. If it takes 38.56 mL of 0.05004 M NaOH to titrate (neutralize) 50.00 mL of H2SO4, what is the concentration of the acid?
This is a type of Solution Stoichiometry also called a Titration.
Titration calculations are fairly straightforward when the reactants are in a one-to-one mole ratio in the balanced chemical reaction, but sometimes they are not, like in this reaction.
Balanced reaction: H2SO4(aq) + 2 NaOH(aq) --> 2 H2O(l) + Na2SO4(aq)
Titration is just another way to say "neutralization", but done in such a way that we can analyze the concentration of a solution, sulfuric acid in this case.
The easiest way to answer this is with a formula, the "titration formula":
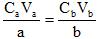
C: Concentration (mol/L) V: Volume (L or mL) subscript a: acid subscript b: base
a: the acid stoichiometric coefficient b: the base stoichiometric coefficient.